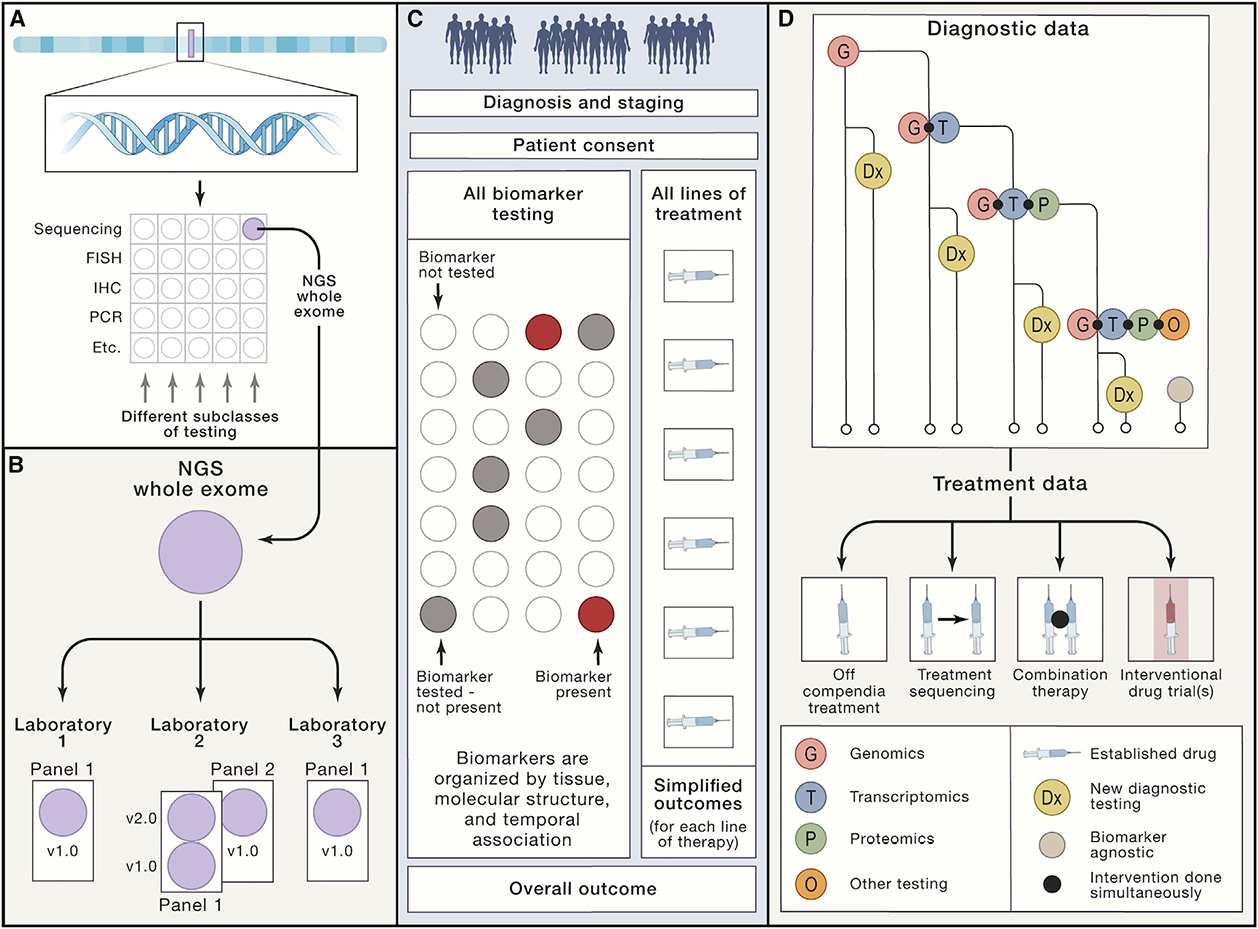
This commentary introduces a new clinical trial construct, the Master Observational Trial (MOT), which hybridizes the power of molecularly based master interventional protocols with the breadth of real-world data. The MOT provides a clinical venue to allow molecular medicine to rapidly advance, answers questions that traditional interventional trials generally do not address, and seamlessly integrates with interventional trials in both diagnostic and therapeutic arenas. The result is a more comprehensive data collection ecosystem in precision medicine.
Introduction
Advances in basic science allow a deeper understanding of the cellular underpinnings of disease. Without clinical correlation, however, many of these molecular techniques take decades to translate into improved patient care. The interplay of genomes, transcriptomes, proteomes, metabolomes, epigenomes, cellular metabolism, microenvironment, immune characteristics, gut biome, and other factors steadily increases the complexity in understanding how everything fits together. A massive obstacle to advancing molecular medicine is the lack of clinical infrastructure to allow exploration, validation, and implementation of the tools needed to turn precision testing into personalized treatment. (Subbiah and Kurzrock, 2018).